Dynalene
Can You Use Galvanized Steel with Glycol-based Heat Transfer Fluids?
Galvanizing is a process by which steel is coated with a thin layer of zinc metal in order to prevent corrosion. The zinc protects the steel both by forming a physical barrier against the environment, and by acting as a sacrificial anode. Zinc has a higher electronegative potential than the iron in steel, so when the two metals are in contact with each other, they form a galvanic cell where the oxidation (corrosion) of the zinc prevents the steel from oxidizing at the same time.
As long as just a part of the zinc coating remains, even portions of the steel that are completely exposed will be more resistant to corrosion than non-galvanized steel. This makes galvanized parts very attractive for many applications – car and bicycle parts, structural components for buildings and bridges, fences and playground equipment, nuts, bolts, and tools, chilled water lines, and many other things can all be found made of galvanized steel.
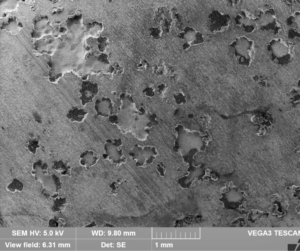
However, galvanized steel is not a recommended material of construction for piping in a system using glycol-based heat transfer fluids. The zinc coating on galvanized steel is meant to prevent corrosion at atmospheric conditions, but the components of a heat transfer system often face much more aggressive conditions. As glycols break down, they form organic acids such as glycolic, glyoxylic, formic, carbonic, and oxalic acids. These acids are corrosive to metals such as iron, steel, and zinc. In systems that run at higher temperatures this breakdown is accelerated.
Phosphate inhibitors are commonly added to glycols for a few reasons. They protect against corrosion caused by these breakdown acids by providing pH buffering, which reduces the activity of the acids. Phosphate also reacts with iron and steel to form a protective coating on the metal surface. Unfortunately, phosphates can react with zinc and form a precipitate. This is bad for galvanized steel for a number of reasons.
As phosphate inhibitor reacts with the zinc coating, the ability of the zinc layer to physically protect the metal beneath is compromised. If the zinc phosphate formed as a new layer on the metal surface, that wouldn’t be so bad – it would just replace one layer with another. But the precipitate that forms comes off of the metal to flow loose in the system, which can cause clogging and subsequent problems with thermal performance.
The reaction also by definition reduces the amount of phosphate present in the fluid. The phosphate ions are not available to coat exposed metal or provide buffering against acids if they’re bound into an insoluble zinc salt. Decreased buffering means that any acid breakdown products that form have the potential to cause more corrosion to the system. Because of all of the above reasons, galvanized steel is not recommended as a material for heat transfer fluid systems.
Text content
- Engineering ToolBox, (2003). Working Pressure Copper Tubes Type K, L and M. [online] Available at: https://www.engineeringtoolbox.com/copper-tubes-dimensions-pressure-d_84.html [Accessed Day Mo. Year]